Activate Ppar
Clinical relevance. PPAR-gamma has been implicated in the pathology of numerous diseases including obesity, diabetes, atherosclerosis, and cancer.
PPAR is one of three members of the soluble nuclear receptor family called peroxisome proliferator-activated receptor PPAR. It is a sensor for changes in levels of.
Nuclear hormone receptors are transcription factors that bind DNA and regulate transcription in a ligand-dependent manner. PPARs Peroxisome Proliferator-Activated Receptors are ligand-inducible transcription factors that belong to the nuclear hormone receptor superfamily, together with the receptors for thyroid hormone, retinoids, steroid hormones and vitamin D that act as ligand-activated transcription factors. PPARs regulate gene expression by binding with RXR Retinoid X Receptor as a heterodimeric partner to specific DNA sequence elements termed PPRE Peroxisome Proliferator Response Element Ref.1. This heterodimeric transcription factor complex then binds to cognate sequences in promoter regions of target genes involved in the catabolism of fatty acids Ref.2. PPAR ligands can be either synthetic, such as peroxisome proliferators, hypolipidaemic drugs, anti-inflammatory or insulin-sensitizing drugs, or endogenous, most of them being fatty acids or their derivatives. PPARs have been implicated in many normal and disease-related biologic processes relevant to the heart and vasculature including lipid and energy metabolism, inflammation, embryo implantation, diabetes and cancer.
There are 3 main isotypes of PPARs, PPAR-Alpha NR1C1, PPAR-Beta also called PPAR-Delta, NUC1 or FAAR, and PPAR-Gamma NR1C3, which are distinguished by tissue- and developmental-specific patterns of expression and by the distinct, overlapping nature of lipid and eicosanoid ligands capable of activating each receptor Ref.3. Although all three isotypes of PPARs have been shown to modulate lipid metabolism, each isotype also has specific functions Ref.4. PPAR-Alpha is highly expressed in tissues with high rates of mitochondrial fatty acid oxidation, such as liver, heart, muscle, kidney and cells of the arterial wall such as monocyte-derived macrophages, smooth muscle and endothelial cells, and it is activated by fibrates, fatty acids and eicosanoids, 15-d Ptg 15-Deoxy-Delta Prostaglandin-J2, and oxidized fatty acids Ref.5. It regulates the expression of genes involved in lipoprotein metabolism, raising levels of apolipoprotein A-1, a major apolipoprotein of HDL High-Density Lipoprotein. PPAR-Gamma is found in adipose tissues, and is activated by fatty acids or their derivatives, that plays a role in insulin sensitivity, adipogenesis and placental function. It activates transcription in concert with coactivators including SRC1 Steroid Receptor Coactivator-1, and has also been implicated in a variety of neoplastic processes, including colorectal cancer. This receptor is the molecular target of TZD Thiazolidinedione class of antidiabetic drugs, which include rosiglitazone and pioglitazone Ref.6. PPAR-Beta is found in most tissues, and is only weakly activated by fatty acids, Ptgs and leukotrienes and has no known physiologically relevant ligand Ref.4. Once activated, PPARs bind to DNA and regulate gene transcription.
The PPAR: RXR heterodimer exists in both an active and inactive state. When inactive, it is bound to corepressors such as the NCOR Nuclear Receptor Corepressor or the SMRT Silencing Mediator For Retinoid and Thyroid Hormone Receptor. In the presence of ligand for either PPAR or RXR, the corepressors dissociate so that the ligand can bind and activate co-activators, such as SRC1 Steroid Receptor Coactivator1, CBP/p300 CREB-Binding Protein, the Tuberous Sclerosis Gene-2 product, the PPAR binding protein, P-GammaC1, P-GammaC2 PPAR-Gamma Coactivator 1 and 2, and Ara70 Ref.3. The only known natural ligand for RXR is 9-cis Retinoic Acid. When the PPAR: RXR complex is activated, it binds to PPRE in the 5 region of target genes to induce transcription. PPAR-Alpha regulates the expression of genes involved in the peroxisomal and mitochondrial Beta-oxidation pathways such as Acyl-CoA oxidase, Enoyl-CoA hydratase/dehydrogenase multifunctional enzyme, Keto-Acyl-CoA thiolase, Malic enzyme, medium chain Acyl-CoA dehydrogenase, and mitochondrial hydroxy methylglutaryl-CoA synthase. PPAR-Alpha also regulates FATP Fatty Acid Transport Protein, the FAT/CD36 Fatty Acid Translocase, L-FABP Liver Cytosolic Fatty Acid-Binding Protein and UCP2 and UCP3 Uncoupling Proteins-2 and 3. By altering transcription of these genes, activated PPAR-Alpha leads to increased breakdown of triglycerides and fatty acids, increased cellular fatty acid uptake, and reduced triglyceride and fatty acid synthesis. The expression of PPAR-Gamma is widespread and it is found at moderate levels in most tissues, with high levels of the protein found in the placenta and large intestine. The activity of PPAR-Alpha and PPAR-Gamma is also regulated by phosphorylation events. Specifically, the recruitment of adaptor molecules, including SHC SH2 containing protein and the GRB2 Growth Factor Receptor-Bound Protein-2 -SOS complex by several growth factor receptors, which leads phosphorylation of Ras and Raf1 molecules. This in turn activates the MAPKs Mitogen Activated Protein Kinases of the ERK Extracellular Signal Regulated Kinase type, which occurs by sequential activation of TAK1 TGF-Beta Activated Kinase-1 and MEKs MAPK/ERK kinases. These kinases inhibit the activities of PPAR-Alpha and PPAR-Gamma. In contrast, GPCR G-Protein Coupled Receptors mediated phosphorylation of PKA Protein Kinase-A by cAMP cyclic Adenosine-3, 5 Monophosphate or p38 MAPK activates PPAR-Alpha. This differential regulation of PPAR activity by signal transduction events provides a mechanism for rapid, cell-specific control of PPAR target gene expression by extracellular stimuli Ref.7.
The PPAR regulatory pathway plays a critical role in the regulation of diverse biologic processes within the cardiovascular system. PPAR-Alpha acts as part of a transcription factor complex that regulates the expression of a number of genes implicated in atherogenesis and plaque stability. Recently, the PPAR-Alpha gene regulatory pathway has been implicated in the hepatic metabolic response to Diabetes Mellitus and PPAR-Alpha ligands such as Wy-14, 643; ciprofibrate and clofibrate have been implicated in peroxisome proliferation and liver tumors. PPAR-Gamma is expressed on all major cells of the vasculature, including endothelial cells, VSMCs Vascular Smooth Muscle Cells and monocytes/macrophages, human coronary artery smooth muscle cells, umbilical artery smooth muscle cells, umbilical endothelial cells, and aortic smooth muscle cells. Mutations that alter the function of PPAR-Gamma cause a syndrome of insulin resistance, hypertension, and dyslipidemia characteristic of the cardiovascular dysmetabolic syndrome Ref.2. Activation of PPAR-Gamma inhibits monocyte and macrophage inflammatory responses by preventing the activation of nuclear transcription factors, such as NF-KappaB Nuclear Factor-KappaB, Activating Protein-1 and STAT1 Signal Transducer and Activator of Transcription-1. Since inflammation plays an important role in atherogenesis, this anti-inflammatory effect of PPAR-Gamma helps to reduce the risk of atherogenesis Ref.4. Recent evidence suggests that PPAR-Gamma ligands have an anti-tumor effect in humans as these compounds decrease cell growth and induce apoptosis in several malignant human cell types, including HCC Hepatocellular Carcinoma, breast adenocarcinoma and colon adenocarcinoma Ref.3. Mutations of PPAR-Gamma results in the development of severe insulin resistant, Type-2 diabetes, hypertension in the absence of obesity, elevated triglycerides and low HDL levels and, a number of components of the metabolic syndrome. PPAR-Delta is a potential downstream target of APC Adenomatous Polyposis Coli /Beta-Catenin/ TCF4 T-Cell Factor-4 tumor suppressor pathway, which is involved in the regulation of growth promoting genes such as c-Myc and Cyclin-D1. PPAR-Beta plays an antiapoptotic role in keratinocytes via transcriptional control of the Akt/PKB Protein Kinase-B signaling pathway. Both PI3K Phosphatidylinositol-3 Kinase and integrin-linked kinase are target genes of PPAR-Beta Ref.3. Many naturally occurring or synthetic compounds are agonists for the PPARs. 15d-Ptg is the most potent endogenous PPAR-Gamma agonist known. Other PPAR-Gamma agonists include the insulin-sensitizing thiazolidinedione family of antidiabetic drugs the glitazones that enhance insulin-mediated glucose transport into adipose and skeletal muscle and are clinically used pharmacological ligands to treat rheumatoid arthritis. Many synthetic compounds such as the NSAIDs Non-Steroid Anti-Inflammatory Drugs are PPAR-Alpha and PPAR-Gamma agonists Ref.4. PPAR-Alpha agonists also include fibric acids, gemfibrozil and fenofibrate that limit cytokine-induced activation of inflammatory functions of VCAM1 in response to TNF-Alpha Tumor Necrosis Factor-Alpha and tissue factor gene expression Ref.2.
Peroxisome proliferator-activated receptor alpha; Identifiers; Symbol: PPARA: Alt. symbols: PPAR: Entrez: 5465: HUGO: 9232: OMIM: 170998: RefSeq: NM_001001928.
PPAR γ induces MUC1-C proteasome-dependent degradation. Figure 2a shows that MUC1-C protein undergoes degradation in the cancer cells HT29, MCF-7 or LS 174T.

Pathway Central: PPAR Pathway
Peroxisome proliferators include hypolipidemic drugs, herbicides, leukotriene antagonists, and plasticizers; this term arises because they induce an increase in the.
Open Badges Research Effects of the PPAR-β agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination.
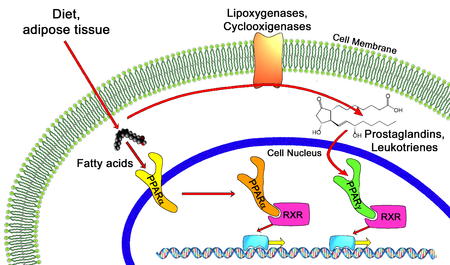
Nuclear hormone receptors are transcription factors that bind DNA and regulate transcription in a ligand-dependent manner. PPARs Peroxisome Proliferator-Activated.
The Peroxisome Proliferator-Activated Receptors PPARs page provides a description of the structure and function of these important regulators of metabolism.